Peerless Info About How To Lower Freezing Point Of Water

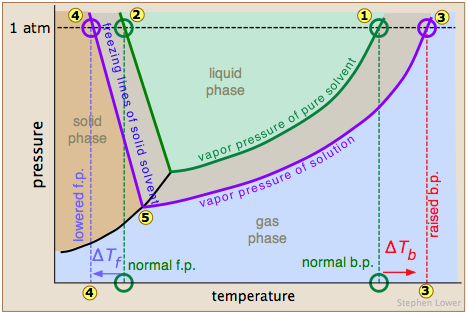
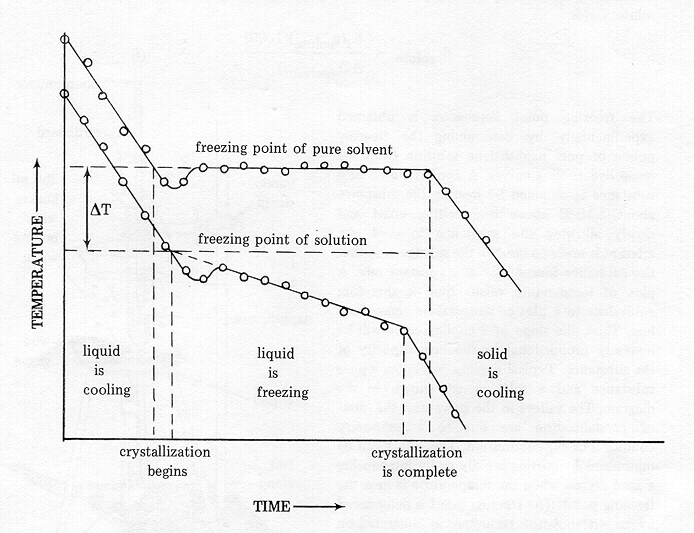
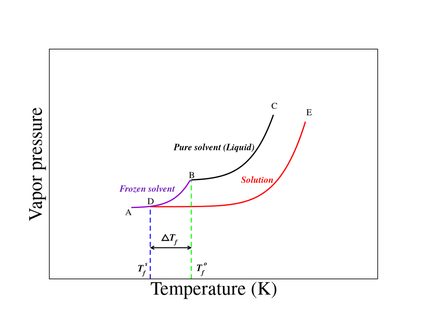
A solution will have a lower freezing point than a pure solvent.
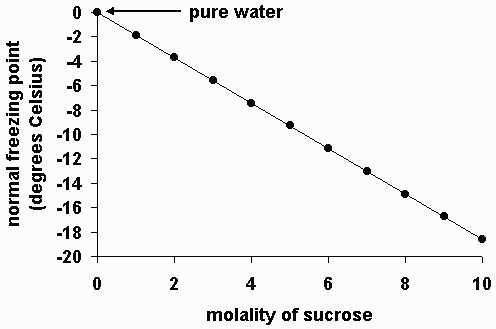
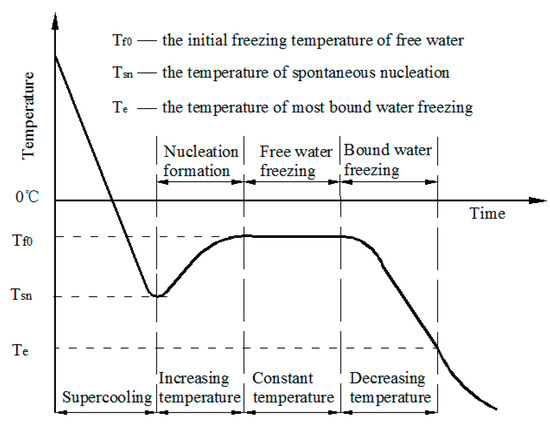
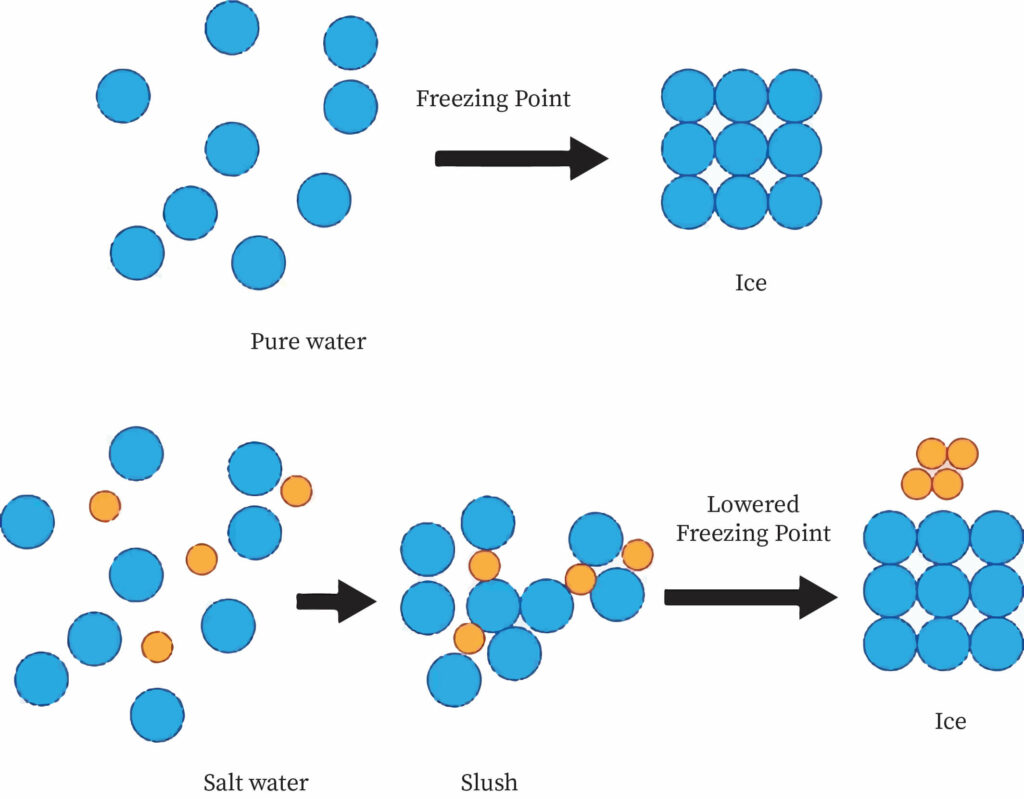
How to lower freezing point of water. Overnight freeze water in multiple different containers. The greater the solute particles there are in a solution, the greater the decrease in freezing temperature. The freezing point of water is simply 0 degrees celsius or 32 degrees fahrenheit.
One of the main reasons for the lower freezing point of alcohol is the fact that it is a much smaller molecule than water. That's why you rarely see. This is called the eutectic point.
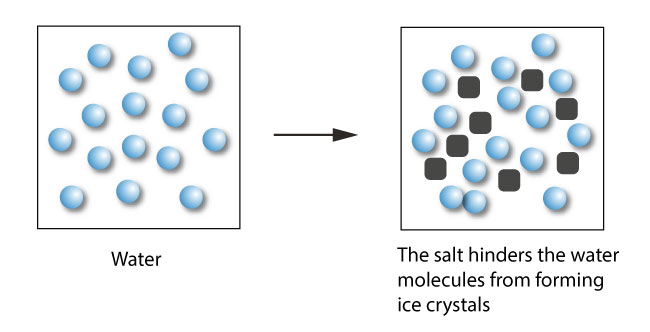
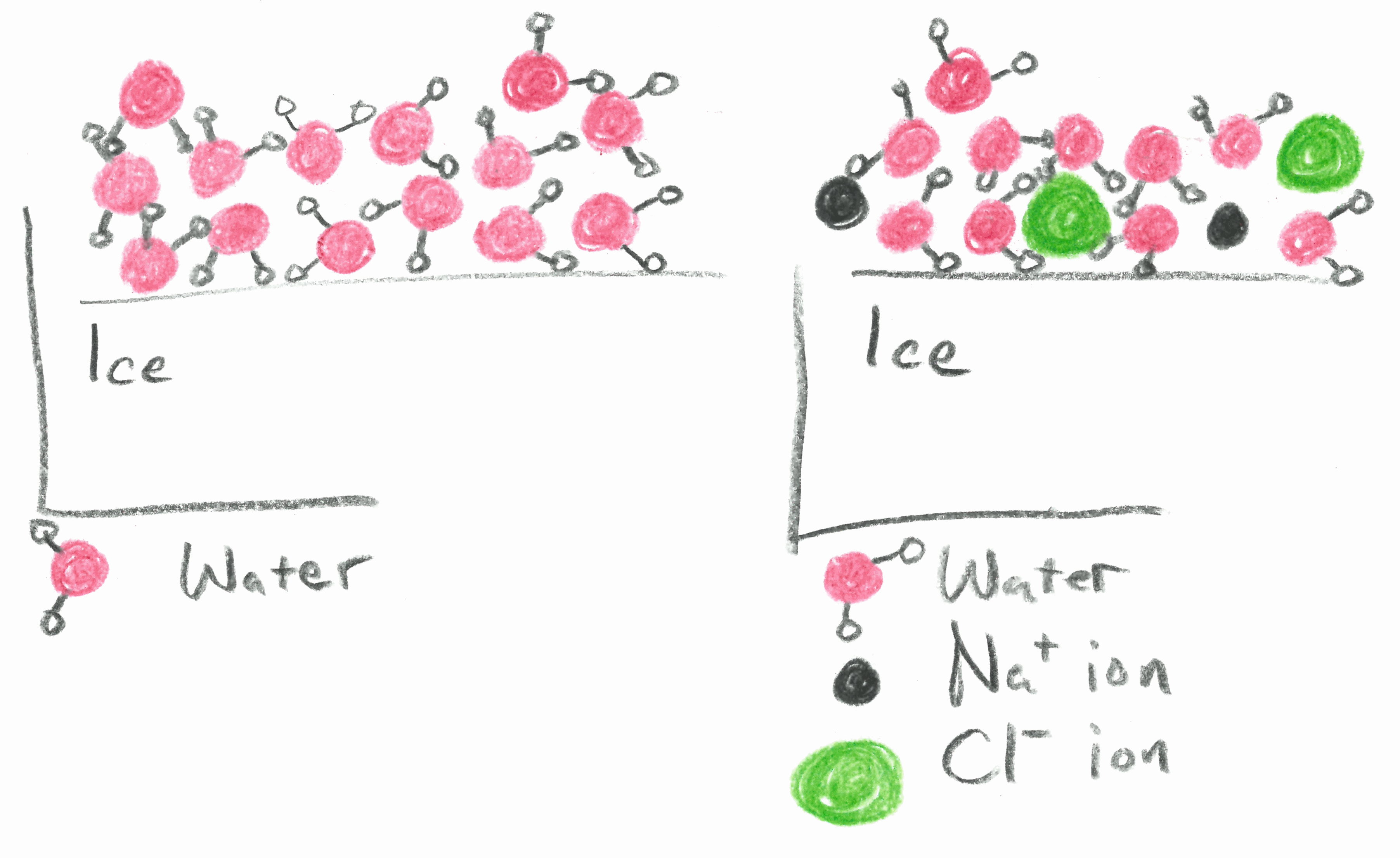
Salt lowers the freezing point of water because it forms ice crystals. So zero degree fahrenheit is the lowest temperature to which you can lower the melting point of water with salt, salt is not the only way to lower the freezing point. Any lower than that, and water must freeze.
But in a study published nov. If 10 grams of sodium chloride were added to 100 grams of water, the freezing. These ice crystals act as nucleation sites for other ice crystals to form.
A deicer is a substance that melts or prevents the formation of ice, and does so by lowering the freezing point of water and preventing a bond between ice and paved surfaces. Add salt to ice and watch it melt away. 30 in the journal nature.
Until now, it was believed that this range stopped at minus 36 f (minus 38 c); Adding an impurity to a solvent alters its physical properties through the combined effects of boiling point elevation and freezing point depression. The first exception is when the solute stops dissolving.

/the-freezing-point-of-water-609418_FINAL-01f50f5f4f7d4a39854bebcc59df1aa4.gif)